News
- Shipments of First Commercial-Use CellFX Systems to
- First Aesthetic Dermatology Patients Treated in the
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210216005479/en/
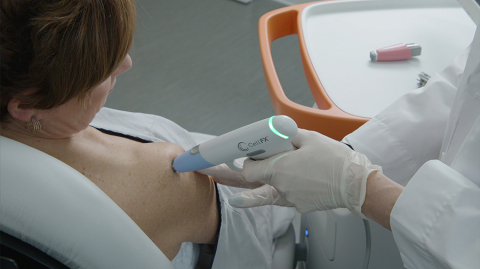
The CellFX System for cellular-based skin conditions (Photo:
As the first-of-its-kind multi-application platform powered by NPS technology delivering nano-second pulses of electrical energy to non-thermally clear cells while protecting adjacent non-cellular healthy tissue, the CellFX System represents an advanced, next-generation modality for dermatology procedures addressing common skin conditions.
“I am thrilled and honored to perform the first commercial CellFX procedure worldwide. Building on our positive clinical research, it is most gratifying to be the first dermatologist to offer my everyday patients a better option to clear a variety of benign lesions,” said
The prevalence of benign cellular skin disorders among patients visiting aesthetic dermatologists today is widespread. Based on a 2019 survey of
“We are excited to announce the start of our controlled launch program and the first ever commercial use of the CellFX System. The introduction of this differentiated procedure gives physicians a new non-thermal, cellular-specific mechanism that is expected to greatly advance how even the most common and frustrating skin conditions are treated,” said
“The ability to execute our controlled launch program simultaneously in two geographies within weeks of their respective regulatory clearances is a testament to the exceptional preparation by our entire organization,” adds
About
To stay informed about the CellFX System, please visit CellFX.com and sign up for updates.
1 2019 Physician (n=304) and Patient (n=405) surveys conducted by third-party market research firm on behalf of
Forward-Looking Statements
All statements in this press release that are not historical are forward-looking statements, including, among other things, statements relating to Pulse Biosciences’ expectations regarding regulatory clearance and the timing of regulatory filings or approvals, NPS technology including the effectiveness of such technology, the CellFX System including the benefits of the CellFX System and commercialization and adoption of the CellFX System, current and planned future clinical studies and the ability of the Company to execute such studies and results of any such studies, other matters related to its pipeline of product candidates, the Company’s market opportunity and commercialization plans, including the timing and results of the controlled launch in the US, the market for the treatment of certain lesions, the experience of using the CellFX System, future financial performance, and other future events. These statements are not historical facts but rather are based on Pulse Biosciences’ current expectations, estimates, and projections regarding Pulse Biosciences’ business, operations and other similar or related factors. Words such as “may,” “will,” “could,” “would,” “should,” “anticipate,” “predict,” “potential,” “continue,” “expects,” “intends,” “plans,” “projects,” “believes,” “estimates,” and other similar or related expressions are used to identify these forward-looking statements, although not all forward-looking statements contain these words. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and assumptions that are difficult or impossible to predict and, in some cases, beyond Pulse Biosciences’ control. Actual results may differ materially from those in the forward-looking statements as a result of a number of factors, including those described in Pulse Biosciences’ filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20210216005479/en/
Investors:
510.241.1077
IR@pulsebiosciences.com
OR
415.937.5406
philip@gilmartinir.com
Media:
Nadine D. Tosk
504.453.8344
nadinepr@gmail.com or
press@pulsebiosciences.com
Source:
