News
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220511005989/en/
Company Updates
- Increased the overall CellFX System commercial session utilization four week moving average1 during Q1 with decreasing trend in early Q2 due to transition in commercial strategy.
- Appointed new commercial leadership and began initiation of the CellFX System utilization program in May with nine commercial clinics to establish commercial integration best practices. Established program goal for each clinic of 40 commercial sessions per month. Average monthly utilization of the nine participating clinics during Q1 was 14 sessions per month.
- Generated first quarter 2022 revenue of
$444 thousand . - Completed one commercial sale of a CellFX System in the first quarter of 2022.
- Transitioned 10 Controlled Launch Program participants to commercial use in the first quarter totaling 39 commercial conversions at the end of the first quarter. There are 20 clinics remaining in the Controlled Launch program after a total of 11 clinics have opted out as of the end of Q1.
- Met with FDA regarding the Additional Information (AI) letter response to the sebaceous hyperplasia 510(k). Provided additional analysis of the clinical data following the meeting, at FDA’s request, and anticipate further communication prior to any formal response to the AI letter.
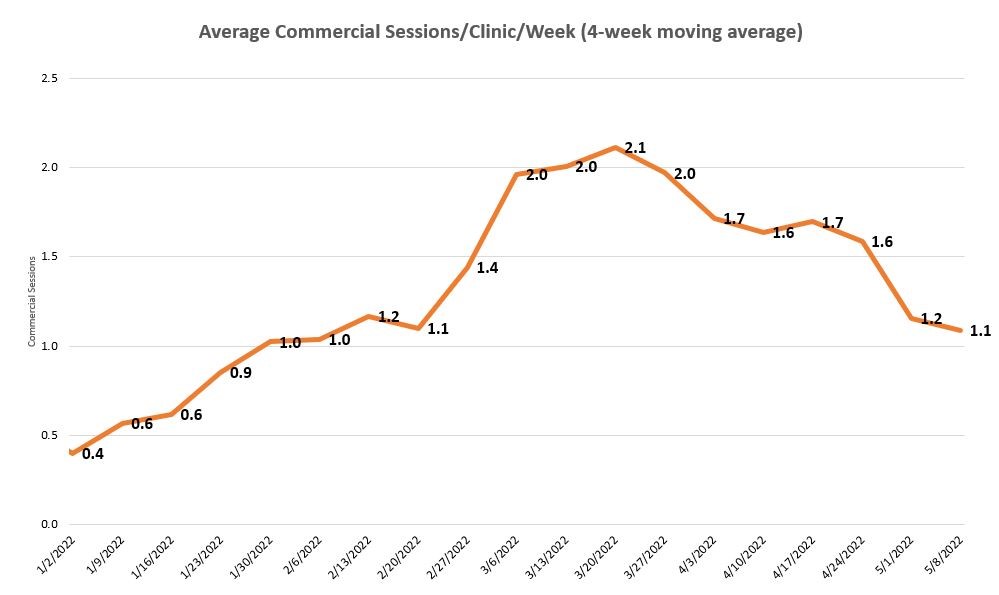
1 Utilization is measured as commercial sessions defined as individual patient treatments, regardless of the number of lesions treated, performed using CellFX Systems that have been purchased or converted to commercial use from the controlled launch program.
“In the first quarter of 2022 we took steps to refocus our CellFX dermatology efforts by bringing in new commercial leadership. We have prioritized increasing CellFX System utilization at a subset of our commercial clinics, with the goal of developing commercial integration best practices that will drive utilization across all clinics. While these best practices are being established there will be a reduced focus on capital sales,” said
First Quarter 2022 Results
Revenue for the three months ended
Total GAAP cost and expenses representing cost of revenues, research and development, sales and marketing and general and administrative expenses for the three months ended
GAAP net loss for the three months ended
Cash, cash equivalents and investments totaled
Reconciliations of GAAP to non-GAAP cost and expenses and net loss have been provided in the tables following the financial statements in this press release. An explanation of these measures is also included below under the heading “Non-GAAP Financial Measures.”
Webcast and Conference Call Information
Pulse Biosciences’ management will host a conference call today,
About
To stay informed about the CellFX System, please visit CellFX.com and sign-up for updates.
Non-GAAP Financial Measures
In this press release, in order to supplement the Company’s condensed consolidated financial statements presented in accordance with Generally Accepted Accounting Principles, or GAAP, management has disclosed certain non-GAAP financial measures for the statement of operations. The Company believes that an evaluation of its ongoing operations (and comparisons of its current operations with historical and future operations) would be difficult if the disclosure of its financial results were limited to financial measures prepared in accordance with GAAP. As a result, the Company is disclosing certain non-GAAP results in order to supplement investors’ and other readers’ understanding and assessment of the Company’s financial performance. Company management uses these measurements as aids in monitoring the Company’s ongoing financial performance from quarter to quarter, and year to year, on a regular basis and for financial and operational decision-making. Non-GAAP adjustments include stock-based compensation, depreciation and amortization and restructuring charges. From time to time in the future, there may be other items that the Company may exclude if the Company believes that doing so is consistent with the goal of providing useful information to management and investors. The Company has provided a reconciliation of each non-GAAP financial measure used in this earnings release to the most directly comparable GAAP financial measure. Investors are cautioned that there are a number of limitations associated with the use of non-GAAP financial measures as analytical tools. Investors are encouraged to review these reconciliations, and not to rely on any single financial measure to evaluate the Company’s business.
Non-GAAP financial measures used by the Company may be calculated differently from, and therefore may not be comparable to, similarly titled measures used by other companies, which could reduce the usefulness of the Company’s non-GAAP financial measures as tools for comparison. Investors and other readers are encouraged to review the related GAAP financial measures and the reconciliation of non-GAAP measures to their most directly comparable GAAP measures set forth below and should consider non-GAAP measures only as a supplement to, not as a substitute for or as a superior measure to, measures of financial performance prepared in accordance with GAAP. Non-GAAP financial measures in this earnings release exclude the following:
Non-cash expenses for stock-based compensation. The Company has excluded the effect of stock-based compensation expenses in calculating the Company’s non-GAAP cost and expenses and net loss measures. Although stock-based compensation is a key incentive offered to employees, the Company continues to evaluate its business performance excluding stock-based compensation expenses. The Company records stock-based compensation expense related to grants of performance and time-based options. Depending upon the size, timing and terms of the grants, as well as the probability of achievement of performance-based awards, this expense may vary significantly but will recur in future periods. The Company believes that excluding stock-based compensation better allows for comparisons from period to period.
Depreciation and amortization. The Company has excluded depreciation and amortization expense in calculating its non-GAAP cost and expenses and net loss measures. Depreciation and amortization are non-cash charges to current operations.
Restructuring charges. The Company has excluded restructuring charges in calculating its non-GAAP cost and expenses and net loss measures. Restructuring programs involve discrete initiatives designed to improve operating efficiencies and include employee termination, contract termination, and other exit costs associated with the restructuring program. The Company believes that excluding discrete restructuring charges allows for better comparisons from period to period.
Forward-Looking Statements
All statements in this press release that are not historical are forward-looking statements, including, among other things, statements relating to Pulse Biosciences’ expectations regarding the Company’s Controlled Launch program and the Company’s other activities to develop and commercialize NPS technology to drive growth, such as statements concerning the timing and prospects for converting participants in the Controlled Launch into commercial customers, statements concerning customer adoption and future use of the CellFX System, and statements concerning the use of best practices to drive utilization across clinics, statements about market opportunities in aesthetic dermatology and in other areas of medicine, statements about potential future regulatory clearances and about expanding the CellFX System’s indications for use, statements relating to the effectiveness of the Company’s NPS technology and the CellFX System to improve patient outcomes, statements relating to the Company’s current and planned future clinical studies and its ability to execute these studies successfully, statements about the Company’s pipeline of product candidates and ability to pursue applications for NPS technology outside of dermatology, statements relating to the impact of COVID-19, statements concerning the impact of the Company’s recent corporate restructuring on its operations, statements about the Company’s rights offering or any other of its future financing activities, and other future events. These forward-looking statements are not historical facts but rather are based on Pulse Biosciences’ current expectations, estimates, and projections regarding Pulse Biosciences’ business, operations and other similar or related factors. Words such as “may,” “will,” “could,” “would,” “should,” “anticipate,” “predict,” “potential,” “continue,” “expects,” “intends,” “plans,” “projects,” “believes,” “estimates,” and other similar or related expressions are used to identify these forward-looking statements, although not all forward-looking statements contain these words. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and assumptions that are difficult or impossible to predict and, in some cases, beyond Pulse Biosciences’ control. Actual results may differ materially from those in the forward-looking statements as a result of a number of factors, including those described in Pulse Biosciences’ filings with the
|
|
||||||||
|
Condensed Consolidated Balance Sheets |
||||||||
|
(In thousands, except per share amounts) |
||||||||
|
(Unaudited) |
||||||||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
||||
|
|
|
2022 |
|
2021 |
||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ |
12,676 |
|
$ |
28,614 |
|
||
|
Accounts Receivable |
21 |
|
61 |
|
||||
|
Inventory |
7,487 |
|
5,824 |
|
||||
|
Prepaid expenses and other current assets |
1,979 |
|
2,131 |
|
||||
|
Total current assets |
|
22,163 |
|
|
36,630 |
|
||
|
Property and equipment, net |
2,554 |
|
2,462 |
|
||||
|
Intangible assets, net |
3,050 |
|
3,216 |
|
||||
|
|
2,791 |
|
2,791 |
|
||||
|
Right-of-use assets |
8,611 |
|
8,785 |
|
||||
|
Other assets |
365 |
|
365 |
|
||||
|
Total assets |
$ |
39,534 |
|
$ |
54,249 |
|
||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ |
3,486 |
|
$ |
2,904 |
|
||
|
Accrued expenses |
4,604 |
|
4,389 |
|
||||
|
Deferred revenue |
16 |
|
16 |
|
||||
|
Lease liability, current |
799 |
|
774 |
|
||||
|
Note payable, current |
— |
|
436 |
|
||||
|
Total current liabilities |
|
8,905 |
|
|
8,519 |
|
||
|
Lease liability, less current |
9,833 |
|
10,040 |
|
||||
|
Total liabilities |
|
18,738 |
|
|
18,559 |
|
||
|
Stockholders’ equity: |
||||||||
|
Preferred stock, |
— |
|
— |
|
||||
|
Common stock, |
29 |
|
29 |
|
||||
|
Additional paid-in capital |
274,240 |
|
271,861 |
|
||||
|
Accumulated other comprehensive income (loss) |
— |
|
— |
|
||||
|
Accumulated deficit |
(253,473 |
) |
(236,200 |
) |
||||
|
Total stockholders’ equity |
|
20,796 |
|
|
35,690 |
|
||
|
Total liabilities and stockholders’ equity |
$ |
39,534 |
|
$ |
54,249 |
|
||
|
|
|||||||||
|
Condensed Consolidated Statements of Operations and Comprehensive Loss |
|||||||||
|
(In thousands, except per share amounts) |
|||||||||
|
(Unaudited) |
|||||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
Three-Month Periods Ended |
|
||||||
|
|
|
|
|
||||||
|
|
|
2022 |
|
2021 |
|
||||
|
Revenues: |
|||||||||
|
Product revenues |
$ |
444 |
|
$ |
— |
|
|||
|
Total revenues |
444 |
|
|
— |
|
||||
|
Cost and expenses: |
|||||||||
|
Cost of revenues |
909 |
|
— |
|
|||||
|
Research and development |
6,769 |
|
9,063 |
|
|||||
|
Sales and marketing |
5,541 |
|
4,146 |
|
|||||
|
General and administrative |
4,498 |
|
5,316 |
|
|||||
|
Total cost and expenses |
|
17,717 |
|
|
18,525 |
|
|||
|
Loss from operations |
(17,273 |
) |
(18,525 |
) |
|||||
|
Other income (expense): |
|||||||||
|
Interest income (expense), net |
|
— |
|
|
(114 |
) |
|||
|
Total other income (expense) |
— |
|
(114 |
) |
|||||
|
Net loss |
|
(17,273 |
) |
|
(18,639 |
) |
|||
|
Other comprehensive gain (loss): |
|||||||||
|
Unrealized gain (loss) on available-for-sale securities |
— |
|
1 |
|
|||||
|
Comprehensive loss |
$ |
(17,273 |
) |
$ |
(18,638 |
) |
|||
|
Net loss per share: |
|||||||||
|
Basic and diluted net loss per share |
$ |
(0.58 |
) |
$ |
(0.71 |
) |
|||
|
Weighted average shares used to compute net loss per common share — basic and diluted |
|
29,745 |
|
|
26,072 |
|
|||
|
|
|
|
|
|
|
|
|
||
|
Three-Month Periods Ended |
|||||||||
|
|
|||||||||
|
Stock Based Compensation Expense: |
2022 |
|
2021 |
||||||
|
Cost of revenues |
$ |
90 |
|
$ |
— |
|
|||
|
Research and development |
457 |
|
3,166 |
|
|||||
|
Sales and marketing |
454 |
|
1,761 |
|
|||||
|
General and administrative |
1,006 |
|
2,038 |
|
|||||
|
Total stock-based compensation expense |
$ |
2,007 |
|
$ |
6,965 |
|
|||
|
|
|
||||||||||
|
Consolidated Revenue Financial Highlights |
|
||||||||||
|
(In thousands) |
|
||||||||||
|
(Unaudited) |
|
||||||||||
|
Three-Month Periods Ended |
|||||||||||
|
|
|||||||||||
|
2022 |
|
2021 |
|||||||||
|
Revenue by category: |
|||||||||||
|
Systems |
$ |
367 |
83% |
$ |
— |
- |
|||||
|
Cycle units |
77 |
17% |
— |
- |
|||||||
|
Total revenue |
$ |
444 |
100% |
$ |
— |
- |
|||||
|
|
|||||||||||
|
Revenue by geography: |
|
||||||||||
|
|
$ |
312 |
70% |
$ |
— |
- |
|||||
|
Rest of World |
132 |
30% |
— |
- |
|||||||
|
Total revenue |
$ |
444 |
100% |
$ |
— |
- |
|||||
|
Reconciliation of GAAP to Non-GAAP Financial Measures |
||||||||
|
The following table presents the reconciliation of non-GAAP financial measures to the most directly comparable GAAP financial measures: |
||||||||
|
(In thousands) |
||||||||
|
(Unaudited) |
||||||||
|
Three-Month Periods Ended |
||||||||
|
|
|
|||||||
|
|
2022 |
|
|
|
2021 |
|
||
|
Reconciliation of GAAP to non-GAAP Cost of revenues: |
||||||||
|
GAAP Cost of revenues |
$ |
909 |
|
$ |
— |
|
||
|
Less: Stock-based compensation expense |
(90 |
) |
— |
|
||||
|
Less: Depreciation and amortization |
(5 |
) |
— |
|
||||
|
Less: Restructuring |
(19 |
) |
— |
|
||||
|
Non-GAAP Cost of revenues |
$ |
795 |
|
$ |
— |
|
||
|
Reconciliation of GAAP to non- |
||||||||
|
|
$ |
6,769 |
|
$ |
9,063 |
|
||
|
Less: Stock-based compensation expense |
(457 |
) |
(3,166 |
) |
||||
|
Less: Depreciation and amortization |
(59 |
) |
(39 |
) |
||||
|
Less: Restructuring |
(127 |
) |
— |
|
||||
|
|
$ |
6,126 |
|
$ |
5,858 |
|
||
|
Reconciliation of GAAP to non-GAAP Sales and marketing: |
||||||||
|
GAAP Sales and marketing |
$ |
5,541 |
|
$ |
4,146 |
|
||
|
Less: Stock-based compensation expense |
(454 |
) |
(1,761 |
) |
||||
|
Less: Depreciation and amortization |
(13 |
) |
— |
|
||||
|
Less: Restructuring |
(546 |
) |
— |
|
||||
|
Non-GAAP Sales and marketing |
$ |
4,528 |
|
$ |
2,385 |
|
||
|
Reconciliation of GAAP to non-GAAP General and administrative: |
||||||||
|
GAAP General and administrative |
$ |
4,498 |
|
$ |
5,316 |
|
||
|
Less: Stock-based compensation expense |
(1,006 |
) |
(2,038 |
) |
||||
|
Less: Depreciation and amortization |
(249 |
) |
(240 |
) |
||||
|
Less: Restructuring |
(41 |
) |
— |
|
||||
|
Non-GAAP General and administrative |
$ |
3,202 |
|
$ |
3,038 |
|
||
|
Reconciliation of GAAP to non-GAAP Cost and expenses: |
||||||||
|
GAAP Cost and expenses |
$ |
17,717 |
|
$ |
18,525 |
|
||
|
Less: Stock-based compensation expense |
(2,007 |
) |
(6,965 |
) |
||||
|
Less: Depreciation and amortization |
(326 |
) |
(279 |
) |
||||
|
Less: Restructuring |
(733 |
) |
— |
|
||||
|
Non-GAAP Cost and expenses |
$ |
14,651 |
|
$ |
11,281 |
|
||
|
Reconciliation of GAAP to non-GAAP Net loss: |
||||||||
|
GAAP Net loss |
$ |
(17,273 |
) |
$ |
(18,639 |
) |
||
|
Add: Stock-based compensation expense |
2,007 |
|
6,965 |
|
||||
|
Add: Depreciation and amortization |
326 |
|
279 |
|
||||
|
Add: Restructuring |
733 |
|
— |
|
||||
|
Non-GAAP Net loss |
$ |
(14,207 |
) |
$ |
(11,395 |
) |
||
View source version on businesswire.com: https://www.businesswire.com/news/home/20220511005989/en/
Investors:
510.241.1077
IR@pulsebiosciences.com
or
415.937.5406
philip@gilmartinir.com
Media:
Nadine D. Tosk
504.453.8344
nadinepr@gmail.com or
press@pulsebiosciences.com
Source:
