Recently cleared by the FDA for dermatologic procedures requiring ablation and resurfacing of the skin, the CellFX System from Pulse Biosciences, Inc. is launching soon in the US. The multi-application platform harnesses Pulse’s proprietary NPS technology to deliver nano-second pulses of electrical energy to non-thermally clear cells while sparing adjacent noncellular tissue.
Ahead, Brenda LaTowsky, MD, a clinical trial investigator for the NPS technology, talks about its efficacy and likely role in her practice.

WHAT IS CELLFX AND WHY ARE YOU ENTHUSIASTIC ABOUT THIS NEW TECHNOLOGY?
Brenda LaTowsky, MD: CellFX is exciting because the technology works in a very novel way. Nano-Pulse Stimulation technology or NPS delivers non-thermal energy in ultra-fast rates to the skin. In doing so, the treatment only affects cells in the skin; it treats the cellular component and leaves the rest of the dermis and epidermis safe and unharmed. It does not affect collagen in the dermis, for example.
We participated in two comparative studies and an optimization study for NPS. In the data released by the company thus far, we have seen efficacy as high as 79 percent clearance rate for common and flat warts (non-genital). It’s interesting to note that over a third of these warts had failed previous modalities, and 71 percent completely cleared with one to two sessions, including the recalcitrant warts.

In trials for sebaceous hyperplasia (SH), initial data showed that 99.5 percent of facial SH lesions treated with NPS energy were rated as clear or mostly clear by clinical investigators 60 days after treatment. Interim results from a larger second study showed that significantly lower energy settings can still achieve high clearance efficacy while lowering the rate of transient skin effects.
I think the sky’s the limit with this new way of treating spots and bumps in the skin. I’m really excited to see what other types of lesions we can treat with this technology. I do know that the company is also testing and evaluating NPS for acne and other lesions, like syringomas and nevi. In a study of seborrheic keratosis, 82 percent of lesions were rated clear or mostly clear 106 days after a single treatment.
Another potential clinical application for this technology currently under investigation is to treat basal cell skin cancers in a non-invasive way. So far, the company has shared findings from a study that shows biopsy-confirmed absence of residual BCC in the known NPS treatment zone.
Many patients come into the dermatology clinic very concerned about benign lesions, such as sebaceous hyperplasia, which affect their skin cosmetically, and warts, which are bothersome. In most cases, these lesions are not harmful to the patient. Because they don’t usually cause serious pain, impact function, or otherwise “medically require” treatment, these concerns are considered “cosmetic.” And these presentations are hard to treat; there have been no easy, effective, and completely safe ways to treat these lesions. I’m thrilled that the CellFX System delivering NPS technology is going to be an additional clinical option to treat lesion types that are concerning to patients—especially cosmetically—but pose a challenge to treat.
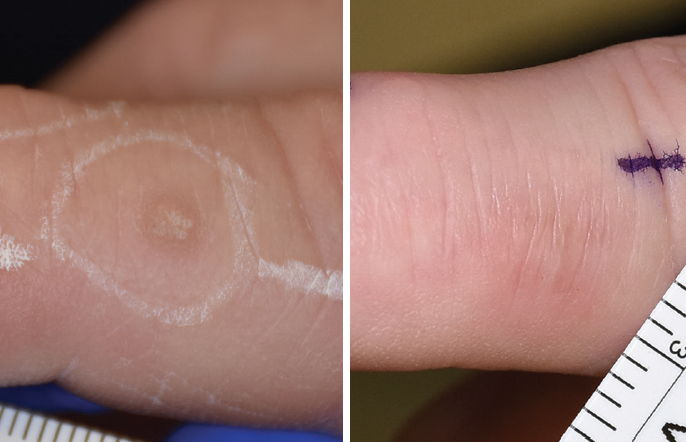
A wart at baseline and after treatment with CellFX NPS technology.
Courtesy of Dr. Gilly Munavalli
WHAT DO YOU FORESEE AS THE ROLE FOR CELLFX IN YOUR PRACTICE?
Dr. LaTowsky: I think the CellFX procedure is going to be great for patients who have warts, SH, and other cosmetic concerns. Like I mentioned, these concerns are hard to treat, and sometimes the current treatments have side effects. This is another option that we can promote to attract new patients to our practice. We can also promote within our practice to attract our current patients who are eager for a new treatment for these spots. It has the appeal of being very straightforward to use, and therefore, I could see this device being delegated to someone under physician supervision.
So far in the office, my staff has been doing all the prep work until the treatment, and I’ve been doing the treatment myself. As such, the staff will clean the area, consent the patient, and I then go through any questions about the consent with the patient to complete the process. Next, I will administer the treatment myself. But in the future I see this being delegated to someone who has been well trained. After the treatment, the staff will do the wound care as well. So really the only thing I have to do is make sure that the settings are entered properly and administer the treatment, which in itself is very brief; the actual procedure does not take more than a few minutes.
The device is compact and runs on standard electrical current. The only consumable is the single-use treatment tip.
HOW DO YOU EXPLAIN THIS TECHNOLOGY TO PATIENTS IN A WAY THAT THEY UNDERSTAND? WHAT IS TREATMENT LIKE?
Dr. LaTowsky: I usually say that NPS technology is using really fast energy that goes into the skin; it targets the spot we want to treat in the skin, and it leaves the rest of the skin untouched and safe.
The treatment, as I mentioned, is really straightforward. Usually we just cleanse the skin; we ask the patients with spots on their face to come in with no makeup. Then we cleanse the skin with an alcohol swab, we enter the settings, and we treat the patient. We use local anesthesia, such as lidocaine, to anesthetize the spot before we treat it. That will make the patient really comfortable during the treatment in most cases.
The majority of patients in the trials were comfortable with local anesthesia in place. As with any treatment, some patients experienced some discomfort. There can be some muscle twitching during the treatment, depending on size and location of the lesion/s, which we always advise the patient of in advance.
Our goal is to remove the lesion with one treatment. With warts or sebaceous hyperplasia, the gold standard out there—electrodesiccation for sebaceous hyperplasia and cryosurgery for warts—often requires more than one treatment. We’re still optimizing the CellFX settings; hence, all the clinical data that’s coming out. I’m enthusiastic that many spots will resolve with one treatment. However, in some cases, there may be the need for a follow-up treatment with the CellFX. As part of setting expectations, we do tell patients that more than one treatment may be necessary, again depending on lesion type and skin type.
After treatment, there is crusting, and a scab forms. We use routine wound care for that scab—basically bland ointment and an elastic bandage. After about a week, the scabs come off and the lesion comes off with it. Because it is non-invasive, there is no inherent risk for infection. Patients should not pick or manipulate the area, and I advise patients to avoid sun exposure to the area for a few weeks after the scab sloughs off, since this is newly healed skin.
As with many treatments that we do in dermatology, there is risk of post-inflammatory erythema or post-inflammatory hyperpigmentation associated with NPS. In the studies, we saw those side effects as being transient, but we do advise patients on that. In clinical practice, I would treat post-inflammatory erythema with either watchful waiting or the pulsed dye laser. I would treat post-inflammatory hyperpigmentation with hydroquinone.
WHAT HAS PATIENT REACTION BEEN LIKE?
Dr. LaTowsky: Patients have been happy with their results. Importantly, a lot of the spots that we treated in the clinical trials were resistant to standard therapies. So it’s been pretty impressive. Often a patient will come in and say, “This wart has been treated five different times and it’s been treated with several different modalities.” We saw those types of lesions actually resolve with the NPS treatments. Patients have been very pleased.

